 |
RRUFF Home | UA Mineralogy | Caltech Mineralogy | The IMA Mineral List | Login |
Important Update News
The RRUFF Project has been migrated to RRUFF.net. Please update your bookmarks immediately, if you have not done so.
The data on this website is already three years out of date, and the entire website will be taken offline before the end of the year.
We are grateful to NASA for the funding of this effort.

|

|
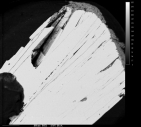
Name: Goethite RRUFF ID: R050142 Ideal Chemistry: FeO(OH) Locality: Park County, Colorado, USA Source: Dave Bunk Minerals Owner: RRUFF Description: Radiating spray of black to brown platy crystals Status: The identification of this mineral has been confirmed by X-ray diffraction and chemical analysis |
| Mineral Group: [ Diaspore (11) ] | ||
| Quick search: [ All Goethite samples (4) ] | ||
| CHEMISTRY | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
|
|||||||||
| RAMAN SPECTRUM | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||
| BROAD SCAN WITH SPECTRAL ARTIFACTS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||
| INFRARED SPECTRUM (Attenuated Total Reflectance) | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||
| POWDER DIFFRACTION | ||||||||
|---|---|---|---|---|---|---|---|---|
| RRUFF ID: | R050142.1 | |||||||
| Sample Description: | Powder | |||||||
| Cell Refinement Output: |
a: 9.9613(2)Å b: 3.0226(2)Å c: 4.6017(3)Å alpha: 90.° beta: 90.° gamma: 90.° Volume: 138.555(9)Å3 Crystal System: orthorhombic |
|||||||
|
|
|||||||
| REFERENCES for Goethite | |
|---|---|
|
American Mineralogist Crystal Structure Database Record: [view record] |
|
|
Anthony J W, Bideaux R A, Bladh K W, and Nichols M C (1990) Handbook of Mineralogy, Mineral Data Publishing, Tucson Arizona, USA, by permission of the Mineralogical Society of America. [view file] |
|
|
Lenz J G (1806) Göthit, in Tabellen über das gesammte Mineralreich Göpferdts Jena 46-46 |
|
|
Posnjak E, Merwin H E (1919) The hydrated ferric oxides, American Journal of Science, 47, 311-348 [view file] |
|
|
Spencer L J (1919) Mineralogical characters of turite (=turgite) and some other iron-ores from Nova Scotia, Mineralogical Magazine, 18, 339-348 [view file] |
|
|
Hoppe W (1940) Über die kristallstruktur von α-AlOOH (diaspor) und α-FeOOH (nadeleisenerz), Zeitschrift für Kristallographie, 103, 73-89 [view file] |
|
|
Bernal J D, Dasgupta D R, Mackay A L (1959) The oxides and hydroxides of iron and their structural inter-relationships, Clay Minerals Bulletin, 4, 15-30 |
|
|
Gorton A T, Bitsianes G, Joseph T L (1965) Thermal expansion coefficients for iron and its oxides from X-ray diffraction measurements at elevated temperatures, Transactions of the Metallurgical Society of AIME, 233, 1519-1525 |
|
|
Szytula A, Burewicz A, Dimitrijevic Z, Krasnicki S, Rzany H, Todorovic J, Wanic A, Wolski W (1968) Neutron Diffraction Studies of α-FeOOH, Physica Status Solidi, 26, 429-434 |
|
|
Cech F, Johan Z (1969) Identité de l´allcharite et de la goethite, Bulletin de la Société Française de Minéralogie et de Cristallographie, 92, 99-100 [view file] |
|
|
International Mineralogical Association (1980) International Mineralogical Association: Commission on new minerals and mineral names, Mineralogical Magazine, 43, 1053-1055 [view file] |
|
|
Schwertmann U, Murad E (1983) Effect of pH on the formation of goethite and hematite from ferrihydrite, Clays and Clay Minerals, 31, 277-284 [view file] |
|
|
Wolska E, Schwertmann U (1989) Nonstoichiometric structures during dehydroxylation of goethite, Zeitschrift für Kristallographie, 189, 223-237 [view file] |
|
|
Cornell R M, Giovanoli R (1991) Transformation of akaganéite into goethite and hematite in the presence of Mn, Clays and Clay Minerals, 39, 144-150 [view file] |
|
|
de Faria D L A, Silva S V, de Oliveira M T (1997) Raman microspectroscopy of some iron oxides and oxyhydroxides, Journal of Raman Spectroscopy, 28, 873-878 [link] |
|
|
Oh S J, Cook D C, Townsend H E (1998) Characterization of iron oxides commonly formed as corrosion products on steel, Hyperfine Interactions, 112, 59-65 [link] |
|
|
Gualtieri A F, Venturelli P (1999) In situ study of the goethite-hematite phase transformation by real time synchrotron powder diffraction, American Mineralogist, 84, 895-904 [view file] |
|
|
Shannon R D, Shannon R C, Medenbach O, Fischer R X (2002) Refractive index and dispersion of fluorides and oxides, Journal of Physical and Chemical Reference Data, 31, 931-970 [view file] |
|
|
Frankel R B, Bazylinski D A (2003) Biologically induced mineralization by bacteria, Reviews in Mineralogy and Geochemistry, 54, 95-114 |
|
|
Hamilton V E, McSween H Y, Hapke B (2005) Mineralogy of Martian atmospheric dust inferred from thermal infrared spectra of aerosols, Journal of Geophysical Research, 110, E12006 [link] |
|
|
Yang H, Lu R, Downs R T, Costin G (2006) Goethite, α–FeO(OH), from single–crystal data, Acta Crystallographica, E62, i250-i252 [view file] |
|
|
Faria D L A, Lopes F N (2007) Heated goethite and natural hematite: can Raman spectroscopy be used to differentiate them?, Vibrational Spectroscopy, 45, 117-121 |
|
|
Alvarez M, Sileo E E, Rueda E H (2008) Structure and reactivity of synthetic Co-substituted goethites, American Mineralogist, 93, 584-590 [view file] |
|
|
Kučerová G, Majzlan J, Lalinská-Voleková B, Radková A, Bačík P, Michňová J, Šottník P, Jurkovič L, Klimko T, Steininger R, Göttlicher J (2014) Mineralogy of neutral mine drainage in the tailings of siderite-Cu ores in eastern Slovakia, The Canadian Mineralogist, 52, 779-798 |
|
|
Sobron P, Bishop J L, Blake D F, Chen B, Rull F (2014) Natural Fe-bearing oxides and sulfates from the Rio Tinto Mars analog site: Critical assessment of VNIR reflectance spectroscopy, laser Raman spectroscopy, and XRD as mineral identification tools, American Mineralogist, 99, 1199-1205 |
|
|
Wang M, Chou I, Lu W, de Vivo B (2015) Effects of CH4 and CO2 on the sulfidization of goethite and magnetite: an in situ Raman spectroscopic study in high-pressure capillary optical cells at room temperature, European Journal of Mineralogy, 27, 193-201 |
|
|
Kreissl S, Bolanz R, Göttlicher J, Steininger R, Tarassov, Markl G (2016) Structural incorporation of W6+ into hematite and goethite: A combined study of natural and synthetic iron oxides developed from precursor ferrihydrite and the preservation of ancient fluid compositions in hematite, American Mineralogist, 101, 2701-2715 |
|
|
Negrão L B A, Da Costa M L, Pöllmann H, Horn A (2018) An application of the Rietveld refinement method to the mineralogy of a bauxite-bearing regolith in the Lower Amazon, Mineralogical Magazine, 82, 413-431 |
|
|
Voelz J L, Arnold W A, Penn R L (2018) Redox-induced nucleation and growth of goethite on synthetic hematite nanoparticles, American Mineralogist, 103, 1021-1029 |
|
|
Markl G, Keim M F, Bayerl R (2019) Unusual mineral diversity in hydrothermal vein-type deposit: The Clara mine, SW Germany, as a type example, The Canadian Mineralogist, 57, 427-456 |
|
|
Heaney P J, Oxman M J, Chen S A (2020) A structural study of size-dependent lattice variation: In situ X-ray diffraction of the growth of goethite nanoparticles from 2-line ferrihydrite, American Mineralogist, 105, 652-663 |
|
|
|