 |
RRUFF Home | UA Mineralogy | Caltech Mineralogy | The IMA Mineral List | Login |
Important Update News
The RRUFF Project has been migrated to RRUFF.net. Please update your bookmarks immediately, if you have not done so.
The data on this website is already three years out of date, and the entire website will be taken offline before the end of the year.
We are grateful to NASA for the funding of this effort.

|
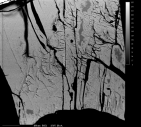
Name: Gypsum RRUFF ID: R040029 Ideal Chemistry: Ca(SO4)·2H2O Locality: Twin Buttes, Pima County, Arizona, USA Source: University of Arizona Mineral Museum 4885 [view label] Owner: RRUFF Description: Colorless prismatic crystal Status: The identification of this mineral has been confirmed by X-ray diffraction. |
|
| Mineral Group: [ Gypsum (12) ] | ||
| Quick search: [ All Gypsum samples (6) ] | ||
| CHEMISTRY | ||||||
|---|---|---|---|---|---|---|
|
|
|||||
| RAMAN SPECTRUM | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||
| BROAD SCAN WITH SPECTRAL ARTIFACTS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||
| INFRARED SPECTRUM (Attenuated Total Reflectance) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| POWDER DIFFRACTION | ||||||||
|---|---|---|---|---|---|---|---|---|
| RRUFF ID: | R040029.1 | |||||||
| Sample Description: | Powder | |||||||
| Cell Refinement Output: |
a: 5.6759(4)Å b: 15.210(1)Å c: 6.5265(6)Å alpha: 90° beta: 118.463(5)° gamma: 90° Volume: 495.33(5)Å3 Crystal System: monoclinic |
|||||||
|
|
|||||||
| REFERENCES for Gypsum | |
|---|---|
|
American Mineralogist Crystal Structure Database Record: [view record] |
|
|
Anthony J W, Bideaux R A, Bladh K W, and Nichols M C (1990) Handbook of Mineralogy, Mineral Data Publishing, Tucson Arizona, USA, by permission of the Mineralogical Society of America. [view file] |
|
|
Agricola G (1546) Gypsum, in De Natura Fossilium translated by Bandy M C, Bandy J A 1955 Geological Society of America New York 89-93 |
|
|
Wallerius J G (1747) in Mineralogia, eller Mineralriket Stockholm [view file] |
|
|
Reusch E (1869) Die Körnerprobe am krystallisirten Gyps, Annalen der Physik, 212, 135-137 [view file] |
|
|
Milton C, Johnston W D (1938) Sulphate minerals of the Comstock Lode, Nevada, Economic Geology, 33, 749-771 [view file] |
|
|
Flörke O W (1952) Kristallographische und röntgenometrische untersuchungen im system CaSO4 - CaSO4·2H2O, Neues Jahrbuch für Mineralogie, Abhandlungen, 4, 180-240 [view file] |
|
|
Papezik V S, Fong C C K (1975) Howlite and ulexite from the carboniferous gypsum and anhydrite beds in western Newfoundland, The Canadian Mineralogist, 13, 370-376 [view file] |
|
|
Pedersen B F, Semmingsen D (1982) Neutron diffraction refinement of the structure of gypsum, CaSO4·2H2O, Acta Crystallographica, B38, 1074-1077 [view file] |
|
|
Abriel V W (1983) Calcium sulfat subhydrat, CaSO4·0,8H2O, Acta Crystallographica, C39, 956-958 [view file] |
|
|
Lager G A, Armbruster T, Rotella F J, Jorgensen J D, Hinks D G (1984) A crystallographic study of the low-temperature dehydration products of gypsum, CaSO4·2H2O: hemihydrate CaSO4·0.50H2O, and γ-CaSO4, American Mineralogist, 69, 910-918 [view file] |
|
|
Mungall J E, Frape S K, Gigson I L, Kamineni D C (1987) Rare-earth abundances in host granitic rocks and fracture-filling gypsum associated with saline groundwaters from a deep borehole, Atikokan, Ontario, The Canadian Mineralogist, 25, 539-543 [view file] |
|
|
Tazaki K, Mori T, Nonaka T (1992) Microbial jarosite and gypsum from corrosion of Portland cement concrete, The Canadian Mineralogist, 30, 431-444 [view file] |
|
|
Cooper M A, Hawthorne F C (1996) The crystal structure of rapidcreekite, Ca2(SO4)(CO3)(H2O)4, and its relation to the structure of gypsum, The Canadian Mineralogist, 34, 99-106 [view file] |
|
|
Schofield P F, Knight K S, Stretton I C (1996) Thermal expansion of gypsum investigated by neutron powder diffraction, American Mineralogist, 81, 847-851 [view file] |
|
|
Kontoyannis C G, Orkoula M G, Koutsoukos P G (1997) Quantitative analysis of sulfated calcium carbonates using Raman spectroscopy and X-ray powder diffraction, Analyst, 122, 33-38 |
|
|
Sarma L P, Prasad P S R, Ravikumar N (1998) Raman spectroscopic study of phase transitions in natural gypsum, Journal of Raman Spectroscopy, 29, 851-856 |
|
|
Boeyens J C A, Ichharam V V H (2002) Redetermination of the crystal structure of calcium sulphate dihydrate, CaSO4·2H2O, Zeitschrift für Kristallographie. New Crystal Structures, 217, 9-10 |
|
|
Shannon R D, Shannon R C, Medenbach O, Fischer R X (2002) Refractive index and dispersion of fluorides and oxides, Journal of Physical and Chemical Reference Data, 31, 931-970 [view file] |
|
|
Cabral A R, Lehmann B, Kwitko-Ribeiro R, Jones R D, Rocha Filho O G (2003) On the association of palladium-bearing gold, hematite, and gypsum in an Ouro Preto nugget, The Canadian Mineralogist, 41, 473-478 [view file] |
|
|
De la Torre Á G, López-Olmo M G, Álvarez-Rua C, García-Granda S, Aranda M A G (2004) Structure and microstructure of gypsum and its relevance to Rietveld quantitative phase analyses, Powder Diffraction, 19, 240-246 |
|
|
Hamilton V E, McSween H Y, Hapke B (2005) Mineralogy of Martian atmospheric dust inferred from thermal infrared spectra of aerosols, Journal of Geophysical Research, 110, E12006 [link] |
|
|
Lane M D (2007) Mid-infrared emission spectroscopy of sulfate and sulfate-bearing minerals, American Mineralogist, 92, 1-18 [view file] |
|
|
Carbone M, Ballirano P, Caminiti R (2008) Kinetics of gypsum dehydration at reduced pressure: an energy dispersive x-ray diffraction study, European Journal of Mineralogy, 20, 621-627 [view file] |
|
|
Christensen A N, Olesen M, Cerenius Y, Jensen T R (2008) Formation and transformation of five different phases in the CaSO4-H2O system: Crystal structure of the subhydrate β-CaSO4·0.5H2O and soluble anhydrite CaSO4, Chemistry of Materials, 20, 2124-2132 |
|
|
Comodi P, Nazzareni S, Zanazzi P F, Speziale S (2008) High-pressure behavior of gypsum: A single-crystal X-ray study, American Mineralogist, 93, 1530-1537 [view file] |
|
|
Buzgar N, Buzatu A, Sanislav I V (2009) The Raman study on certain sulfates, Annalele Stiintifice ale Universitatii, 55, 5-23 [view file] |
|
|
Jehlička J, Vítek P, Edwards H G M, Hargreaves M D, Čapoun T (2009) Fast detection of sulphate minerals (gypsum, anglesite, baryte) by a portable Raman spectrometer, Journal of Raman Spectroscopy, 40, 1082-1086 |
|
|
Seufert S, Hesse C, Goetz-Neunhoeffer F, Neubauer J (2009) Discrimination of bassanite and anhydrite III dehydrated from gypsum at different temperatures, Zeitschrift für Kristallographie, 30, 447-452 [view file] |
|
|
Hildyard R C, Llana-Fúnez S, Wheeler J, Faulkner D R, Prior D J (2011) Electron backscatter diffraction (EBSD) analysis of bassanite transformation textures and crystal structure produced from experimentally deformed and dehydrated gypsum, Journal of Petrology, 52, 839-856 [view file] |
|
|
Harrison T N (2012) Experimental VNIR reflectance spectroscopy of gypsum dehydration: investigating the gypsum to bassanite transition, American Mineralogist, 97, 598-609 [view file] |
|
|
Robertson K, Bish D (2013) Constraints on the distribution of CaSO4·nH2O phases on Mars and implications for their contribution to the hydrological cycle, Icarus, 223, 407-417 |
|
|
Sabron P, Alpers C N (2013) Raman spectroscopy of efflorescent sulfate salts from Iron Mountain Mine Superfund Site, California, Astrobiology, 13, 270-278 |
|
|
Bishop J L, Lane M D, Dyar M D, King S J, Brown A J, Swayze G A (2014) Spectral properties of Ca-sulfates: gypsum, bassanite, and anhydrite, American Mineralogist, 99, 2105-2115 |
|
|
Lopez-Reyes G, Sobron P, Lefebvre C, Rull F (2014) Multivariate analysis of Raman spectra for the identification of sulfates: Implications for ExoMars, American Mineralogist, 99, 1570-1579 |
|
|
Sobron P, Bishop J L, Blake D F, Chen B, Rull F (2014) Natural Fe-bearing oxides and sulfates from the Rio Tinto Mars analog site: Critical assessment of VNIR reflectance spectroscopy, laser Raman spectroscopy, and XRD as mineral identification tools, American Mineralogist, 99, 1199-1205 |
|
|
Geng G, Myers R J, Kilcoyne A L D, Ha J, Monteiro P J M (2017) Ca L2,3-edge near edge X-ray absorption fine structure of tricalcium aluminate, gypsum, and calcium (sulfo)aluminate hydrates, American Mineralogist, 102, 900-908 |
|
|
Vaniman D T, Martinez G M, Rampe E B, Bristow T F, Blake D F, Yen A S, Ming D W, Rapin W, Meslin P Y, Morookian J M, Downs R T, Chipera S J, Morris R V, Morrison S M, Treiman A H, Achilles C N, Robertson K, Grotzinger J P, Hazen R M, Wiens R C, Sumner D Y (2018) Gypsum, bassanite, and anhydrite at Gale crater, Mars, American Mineralogist, 103, 1011-1020 [view file] |
|
|
Biagioni C, Mauro D, Pasero M (2020) Sulfates from the pyrite ore deposits of the Apuan Alps (Tuscany, Italy): A review, Minerals, 10, 1092 [view file] |
|
|
Theophrastus (315 BC) Gypsum, in De Lapidibus, translated by Eichholz D E, 1965 Clarendon Press Oxford 83-85 [view file] |
|
|
|